Abstract
Background: The abnormal regulation and overexpression of Enhancer of zeste homolog 2 (EZH2) was tested in advanced tumor. EZH2 inhibits the transcription of target genes by trimethylation of lysine 27 on histone H3 (H3K27me3), and its activity-enhancing mutation and abnormal expression are the decisive factor for abnormal transcription in many tumors. Besides, EZH2 significantly correlated with tumor malignancy and poor prognosis. XNW5004 is a class of small-molecule EZH2 inhibitor. Preclinical shows that XNW5004 has good safety and anti-tumor efficacy.
Methods: This Chinese, open-label, single-arm, multicenter, phase I study is evaluating the safety, tolerability, Pharmacokinetics (PK), Pharmacodynamics (PD), and efficacy of XNW5004 in adult subjects with pathologically confirmed relapsed/refractory non-Hodgkin lymphoma (NHL). Eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance score of 0-1, life expectancy ≥12 weeks, and adequate bone marrow, renal, and liver function. The study uses an accelerated titration and traditional 3+3 dose-escalated design, with a cycle of 28 days. This study reports data on pts with dose-escalated phase.
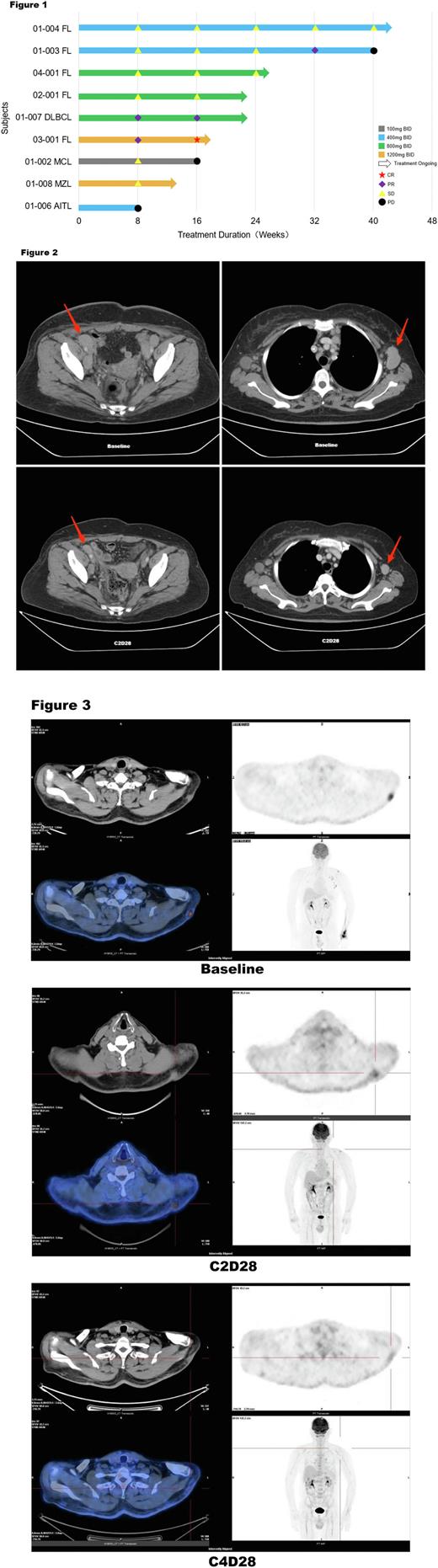
Results: In the dose-escalated phase, as July 15, 2022 , a total of 15 pts were enrolled and treated with 5 dose groups of XNW5004 (from 100 mg to 1600 mg BID). The median age was 58 years (range, 47-71). Pts received a median of 3 (range, 2-7) prior lines of treatment and diagnoses of diffuse large B-cell lymphoma (DLBCL, n = 3), follicular lymphoma (FL, n = 6), mantle cell lymphoma (MCL, n = 1), marginal zone lymphoma (MZL, n = 2), small lymphocytic lymphoma (SLL, n = 1), angioimmunoblastic T-cell lymphoma (AITL, n = 1), and Waldenström macroglobulinemia (WM, n = 1). XNW5004 had a favorable safety and tolerability, and no dose-limiting toxicity (DLT) was observed at doses up to 1600 mg BID. Grade 3 treatment-related adverse events (TRAEs) occurred in 4 (26.7%) pts, including white blood cell decreased, neutrophil count decreased, lymphocyte count decreased, and platelet count decreased. No grade ≥4 adverse events (AEs) and serious TRAEs occurred. Any grade TRAEs in ≥20% of pts included nausea (33.3%), diarrhea (33.3%), alanine aminotransferase increased (26.7%), neutrophil count decreased (26.7%), white blood cell decreased (26.7%), hypertriglyceridemia (26.7%), aspartate aminotransferase increased (20%), vomiting (20%), blood lactate dehydrogenase increased (20%), blood bilirubin increased (20%), pruritus (20%), anemia (20%). With a median follow-up of 4 (range, 2-10) cycles, the objective response rate (ORR) was 33.3%, and the disease control rate (DCR) was 78% in the 9 evaluable pts. The median progression-free-survival (mPFS) and median duration of response (mDOR) were not reached. Among pts with EZH2 wild-type (WT) FL (n=5) , the ORR was 40%, the DCR was 100%, the mPFS and mDOR were not reached. In all pts with stable disease (SD), the lesions continued to shrink. One patient (Figure 2) with DLBCL (non-GCB) received 2 cycles of XNW5004, the lesions were reduced by 59.8%; and after 4 cycles of treatment, the lesions were reduced by more than 70%. One patient (Figure 3) with FL (EZH2 WT) received 2 cycles of XNW5004, the subcutaneous lesions and axillary lymph nodes were significantly reduced, PET-CT showed that uptake was strongly reduced, and the Deauville score (DS) was 5 and 3, respectively. The efficacy evaluation was partial response (PR). After 4 cycles of treatment , PET-CT showed that the metabolism of subcutaneous lesions and axillary lymph nodes continued to decrease, the DS was 2 and 1, respectively, and the efficacy evaluation was complete response (CR). In addition, the PK of XNW5004 was well.
Conclusions: XNW5004 was safety, well tolerated, and efficacy in pts with different types of lymphomas despite high disease burden, with encouraging preliminary anti-lymphoma activity observed. These data support ongoing dose-escalated phase , with multiple cohort expansions at multiple doses. Internal Study identifier: XNW5004-I/II-01.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal